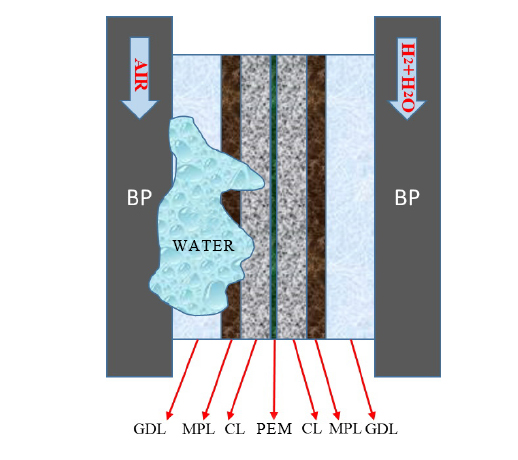
PEMFC(Proton Exchange Membrane Fuel Cell) structure includes BP, GDL(Gas Diffusion Layer), MPL(Micro Porous Layer), CL(Catalyst Layer), and PEM(Proton Exchange Membrane).
The working process of PEMFC mainly includes the following steps.
1. Transport of reactants: During the working process of PEMFC, continuously suppling reactant is necessary to ensure the operation of the fuel cell. In the process of mass transfer, the structure and size design of the flow channel have a huge influence on the performance of the fuel cell.
2. Electrochemical reaction: After the reaction gas transported to the electrode, under the action of catalysis, an electrochemical reaction occurs between hydrogen and oxygen.
3. Conduction of ions (and electrons): After hydrogen is ionized by catalytic action, hydrogen ions and electrons are generated. In the external circuit, electrons are directed from the anode plate to the cathode side. At the same time, hydrogen ions are transported to the cathode side through the polymer membrane, and the oxygen at the cathode is ionized into oxygen ions after obtaining electrons. Oxygen ions and hydrogen ions react to form water.
4. Discharge of water: During the working process of PEMFC, hydrogen and oxygen (air) undergo an electrochemical reaction through catalytic action, and the reaction product is water. If water continues to accumulate, it will seriously affect the transport process of reactants inside the fuel cell, thereby affecting the cell performance. The water generated by the reaction needs to be properly discharged through the flow channel in time.
Compared with lithium battery, fuel cells are developing. LiFePO4 battery has more mature technology and has widely used at golf carts, power wall, energy storage and portable power station. Want know more on this, you can contact with Greenbatt.